Prisma Health technology approved to help tackle ventilator shortage
Staff Report //March 25, 2020//
Prisma Health technology approved to help tackle ventilator shortage
Staff Report //March 25, 2020//
Prisma Health has received emergency use authorization to distribute and use a new technology developed by its physicians to treat four patients simultaneously on a single ventilator.
The VESper device, created by a Prisma emergency medicine physician in collaboration with her husband and a Prisma pulmonary critical care physician, uses “Y” splitter tubing to divide the air flow among multiple patients, according to a news release.
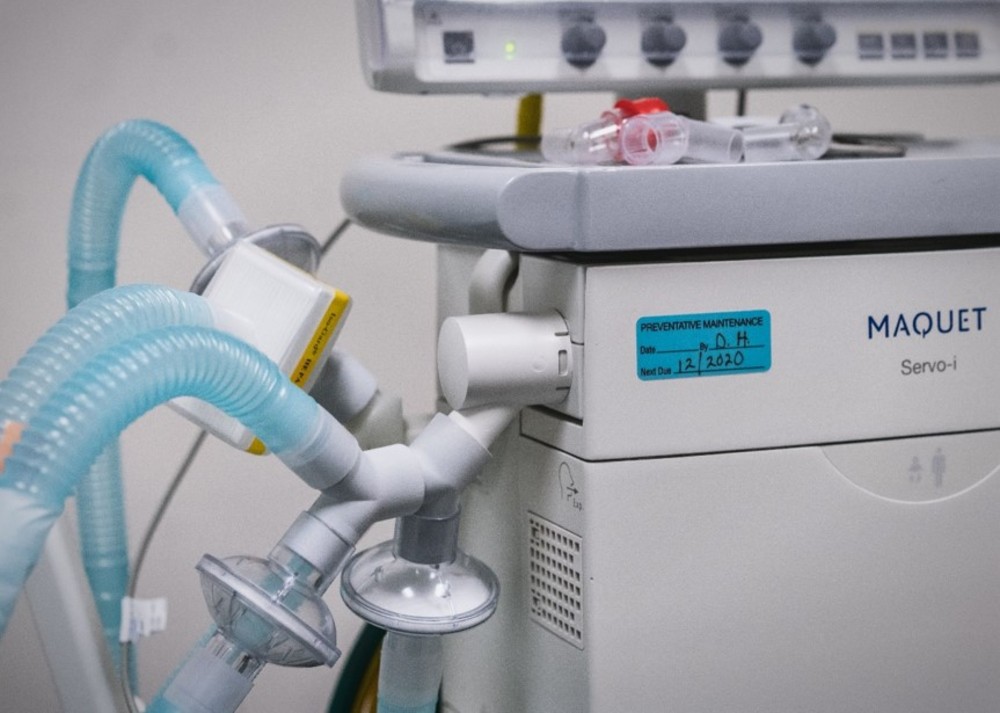 This blueprint was sent to engineers at Clemson University and the University of South Carolina who obtained Food and Drug Administration approval and tested prototypes through 3D printing technology with private sector partners, according to the release.
This blueprint was sent to engineers at Clemson University and the University of South Carolina who obtained Food and Drug Administration approval and tested prototypes through 3D printing technology with private sector partners, according to the release.
After Prisma Health’s Healthcare Simulation Center deemed the device viable for reaching patient breathing parameters, the FDA approved the prototype technology under emergency-use authorization today due to a national ventilator shortage caused by the COVID-19 pandemic. According to the release, HP Inc. and other partners have collaborated with Prisma to create and distribute device parts to the Federal Emergency Management Agency’s COVID-19 “hot spots” and other areas of significant need.
"Immediately, we realized we had an opportunity to impact patient outcomes all over the country, and potentially beyond the U.S.," said Marjorie Jenkins, M.D., chief academic officer for Prisma Health-Upstate and dean of the University of South Carolina School of Medicine Greenville, in the news release. "What we needed was a collaborative team to put the plan in motion and close the loop between design, production, FDA approval and distribution to hospitals with critical need."
Hospitals across the country can register for a free source code and device printing parameters by registering.
"I am so proud of the creativity and perseverance of our clinical team who came together to develop a potentially life-saving solution at a critical time for our country, our communities and our patients.” Mark O'Halla, president and chief executive officer of Prisma Health, said in the release. “We are anxiously awaiting the results of the prototype field tests."
o