COVID-19 vaccines transported by Greenville product
Molly Hulsey //December 22, 2020//
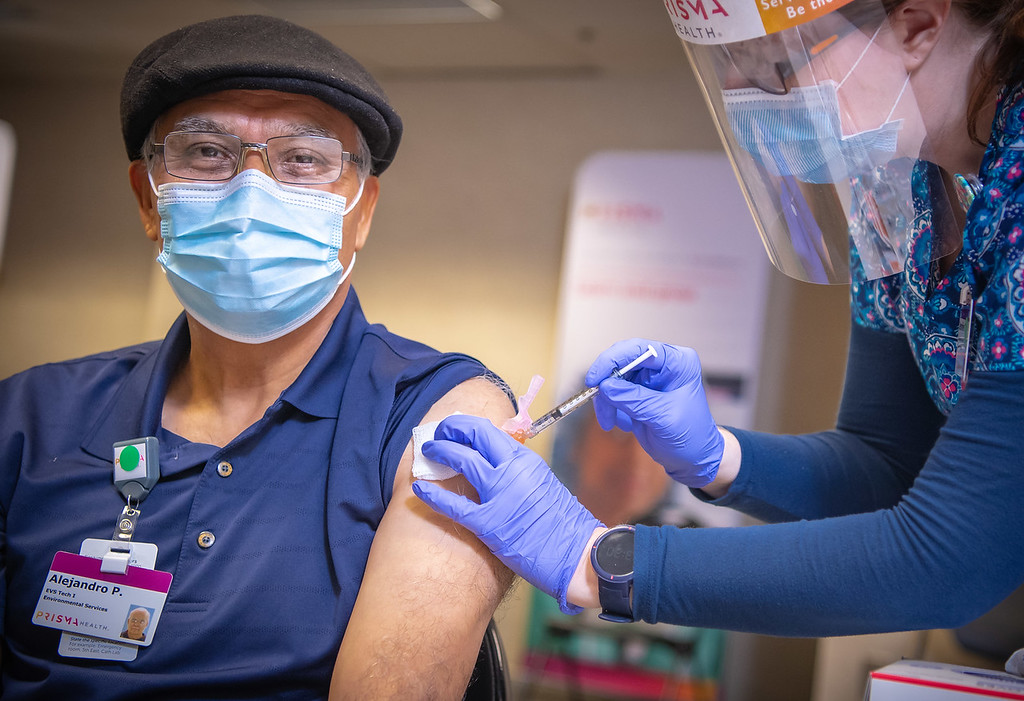
Frontline workers at Prisma Health received the first shipment of Pfizer’s COVID-19 vaccine last week, but to some extent, the shipment was simply returning home to the Upstate.
Greenville is the North American headquarters for Softbox, the British manufacturer of the insulated containers critical to the stability of the Pfizer vaccine.
“I consider this the start to the finish as we move into this process of trying to get enough immunity into the community,” Dr. Steve Shelton said as the dry ice-laden Softbox made a triumphant arrival at Prisma Health-Midlands, complete with pom-poms and cheering health care workers. Shelton is an emergency room physician with Prisma Health who spearheaded COVID-19 treatment efforts in the Midlands.
“I know there is a lot of anxiety about this, but I am confident in the FDA in making sure that they have approved an effective vaccine,” he said. “I feel like I am honored to be here to receive this and am doing my part to combat this disease.”
To remain effective, the vaccine must be shipped at temperatures colder than most of Antarctica, in a box with more layers than a Russian doll. The Softbox includes a top layer or “pod” of dry ice housed on top of five trays of the vaccine, which in turn, nests in a carrying box with a foam lid and temperature gauge. All this is fitted in a cardboard shipping container, according to a Dec. 3 manual from Pfizer.
The vaccine must be stored in an ultra-low temperature freezer long term, but the Softbox Medium ULT Parcel Shipper has been engineered to serve as a temporary storage box if the dry ice is replenished within 24 hours of receiving the box.
With assistance from the U.S. government and state officials, Pfizer laid the groundwork for vaccine distribution from sites in Kalamazoo, Mich., and Pleasant Prairie, Wisc., using a just-in-time system, ensuring that the vaccines were shipped directly to their administration site within one or two days, according to a November Pfizer news release.
The Softbox container is able to maintain the necessary temperatures for up to 10 days if unopened, but to ensure safe transport, Pfizer tracks each shipment 24 hours a day using a GPS-enabled thermal sensor and control tower.
 Only this September, researchers were still scrambling to answer how the vaccine — once approved — could be shipped and stored. Supply chain experts questioned how ultra-low temperature-controlled packaging or TCP manufacturers could match the demand for these specialized products once launched.
Only this September, researchers were still scrambling to answer how the vaccine — once approved — could be shipped and stored. Supply chain experts questioned how ultra-low temperature-controlled packaging or TCP manufacturers could match the demand for these specialized products once launched.
Clive Bryant, global product and marketing director at Softbox, noted in a statement that the commercialization process, temperature limitations and supply chain slowdowns presented a “massive challenge” to fast-tracking vaccine distribution and storage in the fall.
“Current logistical setbacks have clearly constrained the supply chain; land, sea and air transportation have been hit by shrinking capacity, rising costs, delays or a lack of workers due to the coronavirus,” Bryant said in the November statement. “It’s a torrid scenario. The infrastructure powering the global economy is scaling down for protracted downturn just as pharma companies need to scale up for the biggest and most consequential product launch in modern history.”
Due to client confidentially agreements, Softbox executives could not comment on its role in the distribution of the COVID-19 vaccine, but John Hammes, Softbox’s general manager for the Americas, did indicate plans for expanding its Greenville presence.
“We moved into our current 65,000-square-foot custom site in June 2018 and are operating at more than 95% capacity,” Hammes told GSA Business Report in a statement. “We are currently examining opportunities to add another 55,000 square feet. Additionally, we work with off-site vendors for key projects that demand additional space, and at our growth rate, we’re looking at another 100,000 plus square feet in the near future.”
The expansion will also include additional jobs in the Greenville market, a critical nexus for Softbox System’s American distribution web, stretching across North, South and Central America.
Softbox originally moved to Greenville in 2013 after scouting out locations to meet its corporate needs.
With assistance from the Greenville Chamber, SCBio, the Greenville Area Development Corp. and The Furman Group, the company transitioned from a “completely outsourced model” to an in-house operation with manufacturing, production and warehousing capacities, along with a fully-equipped laboratory, Hammes said.
Greenville’s “superior business climate” first attracted the Buckinghamshire-based company to the area, coupled with its skilled workforce and proximity to vendor partners and transportation hubs like the Interstate 85 corridor and ports system, he said.
Softbox also taps Clemson University’s specialized packaging engineers and labs to ensure their products meet testing standards.
 “Our American headquarters gives us tremendous opportunity to recruit talented packaging engineers, executive team members and other professionals,” Hammes said in the statement.
“Our American headquarters gives us tremendous opportunity to recruit talented packaging engineers, executive team members and other professionals,” Hammes said in the statement.
COVID-19 transport may prove to be a boon for Softbox, but it's not its only product featured in recent news coverage. The company’s 100% recyclable cold temperature-based shipping line Tempcell ECO made Fast Company’s list of 2020 “World Changing Ideas” this October after it became available on the American market.
“Pharma, diagnostic and medtech companies, often drivers for change, are setting clear targets to reduce the industry’s environmental footprint,” he said.
Opting for corrugated cardboard instead of the typical plastic casing, Tempcell Eco uses Thermaflute insulation to keep prescription drugs, over-the-counter drugs and animal health products, at 2 degrees Celsius to 25 degrees Celsius 72 hours, according to Hammes.
F